1. Introduction
Patients with FLT3mut acute myeloid leukemia (AML) have higher rates of relapse and a poor prognosis. FLT3 inhibitors and allogeneic stem cell transplant have resulted in improved survival, however, outcomes remain dismal - particularly for FLT3-ITD. Attempts to intensify established regimens are ongoing. The daunorubicin dose in the RATIFY trial was 60 mg/m 2; one common intensification approach in clinical practice is increasing the daunorubicin dose to 90 mg/m 2 in selected patient populations. The aim of this study was to define the baseline characteristics of these patient populations and analyze outcomes stratified by receipt of daunorubicin intensity in FLT3mut AML.
2. Methods
We analyzed 40 patients from the Project ERIS database with newly diagnosed AML treated with 7+3 (daunorubicin and cytarabine) and midostaurin from January 1, 2013 to April 18, 2023 at VCU Massey Comprehensive Cancer Center. All patients had FLT3-ITD or FLT3-TKD. We divided patients into two groups: receipt of daunorubicin 60 mg/m 2 and daunorubicin 90 mg/m 2. We recorded baseline patient-related and disease characteristics, including age, ECOG, Charlson comorbidity index (CCI) scores, molecular profiling and ELN 2022 cytogenetic risk, dates of regimen initiation, and survival. We used the D'Agostino & Pearson method for normality testing and the t-test or Mann-Whitney (as applicable) tests for between-group comparisons. Categorical comparisons used Fischer's exact test. We applied the Bonferroni correction if multiple comparisons were made. The Wilson-Brown method was used for 95% confidence intervals. We analyzed survival by the Kaplan-Meier method with significance determined by the log-rank test. The event for calculating the overall survival (OS) was the date of death. Patients were otherwise censored at the date of last contact.
3. Results
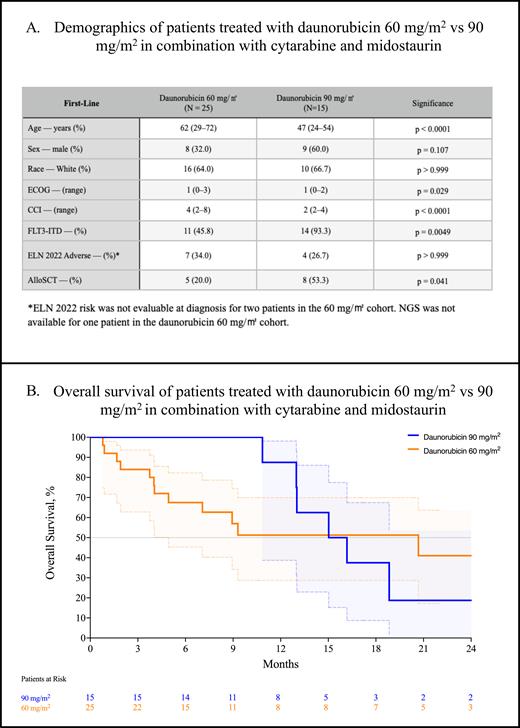
In the overall cohort of 40 patients treated with 7+3 and midostaurin, 25 (62.5%) were treated with daunorubicin 60 mg/m 2 and 15 (37.5%) received daunorubicin 90 mg/m 2. Patients in the 60 mg/m 2 cohort were significantly older compared with the 90 mg/m 2 cohort (62 y. vs 47 y., p < 0.0001, Table A). Patients that received 60 mg/m 2 had worse CCI scores indicating more numerous comorbidities compared with those that received 90 mg/m 2 (4 vs 2, p < 0.0001). While the median ECOG score was 1 in both cohorts, the ECOG scores were significantly worse in the 60 mg/m 2 cohort - however, the significance disappeared after applying the Bonferroni correction (p = 0.029). All but one patient received next-generation testing at diagnosis. Significantly fewer patients in the 60 mg/m 2 cohort had FLT3-ITD (45.8% vs 93.3%, p = 0.0049); the remainder harbored FLT3-TKD. There was no difference in the proportion of ELN 2022 adverse-risk disease between the two cohorts (34.0% vs 26.7%, p > 0.999).
The composite complete response rate (CRc; CR + CRi + CRh) was not significantly different between the two groups: 47.8% (95% CI, 29.2-67.0) for 60 mg/m 2 and 66.7% (95% CI, 41.7-84.8, p = 0.254) for 90 mg/m 2. There was no difference in the rates of MRD negativity between the lower and higher dose groups (18.2%, 95% CI, 3.2-47.7 vs 40.0%, 95% CI, 16.8-68.7, p = 0.254). Significantly more patients in the 90 mg/m 2 cohort proceeded to allogeneic stem cell transplant (53.3% vs 20.0%, p = 0.041). Despite this, we observed no difference in the median overall survival between the 60 mg/m 2 cohort at 20.7 m. and the 90 mg/m 2 cohort at 15.6 m. (p = 0.520, Figure B).
4. Discussion
Individuals in the daunorubicin 60 mg/m 2 cohort were significantly older with worse comorbidities, resulting in significantly fewer patients proceeding to stem cell transplant. Despite this, there was no difference in the rates of response, MRD negativity, or median overall survival when stratified by receipt of daunorubicin intensity. A significantly higher proportion of FLT3-ITD relative to FLT3-TKD in the 90 mg/m 2 cohort may partially explain these findings. These results suggest a dose of daunorubicin 60 mg/m 2 in combination with cytarabine and midostaurin in patients fit for intensive chemotherapy.
Disclosures
Grant:Prescient Therapeutics: Research Funding. Maher:Sobi (Doptelet): Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal